Superconductor – Energy saver for Energy utilization
“Energy can neither be created nor be destroyed, it can only be transformed from one form to another form”
“Entropy of pure crystalline substance at absolute zero temperature is zero”
The statements above are laws of thermodynamics taught during our study in secondary school. Energy garnered from all renewable and non-renewable resources is being transmitted across the continents in the form of Electrical Energy. During its transmission, one-fifth of energy is converted as heat energy due to resistance offered by transmitting lines.
The energy lost as Heat – Can’t it be utilized for some other purpose? Here comes the quality of energy defined by entropy. Energy in the form of mechanical (kinetic or rotational energy has higher entropy and can be converted into some other form for use in applications. Energy converted as heat has low entropy in an open atmosphere, which means its recovery and utilization for applications is near impossible. Rather than trying to utilize the heat energy, an optimistic way to address this issue is to minimize the conversion of energy into heat which accounts for 20%.
Energy loss in the form of heat energy may not be eliminated but can be minimized. The heat generated is given by Joule’s law of heating and is directly proportional to three parameters (i) Square of current (I) passing through the conductor, (ii) Time (t) for which the current passes through the conductor, and (iii) Resistance (R) offered by conducting material. By altering the parameters suitably, heat generated can be minimized. Altering Current (I) and Time (t) are not possible as energy requirements at all places are to be met throughout the year. The only viable option to minimize heat loss then is to minimize the resistance offered by the material.
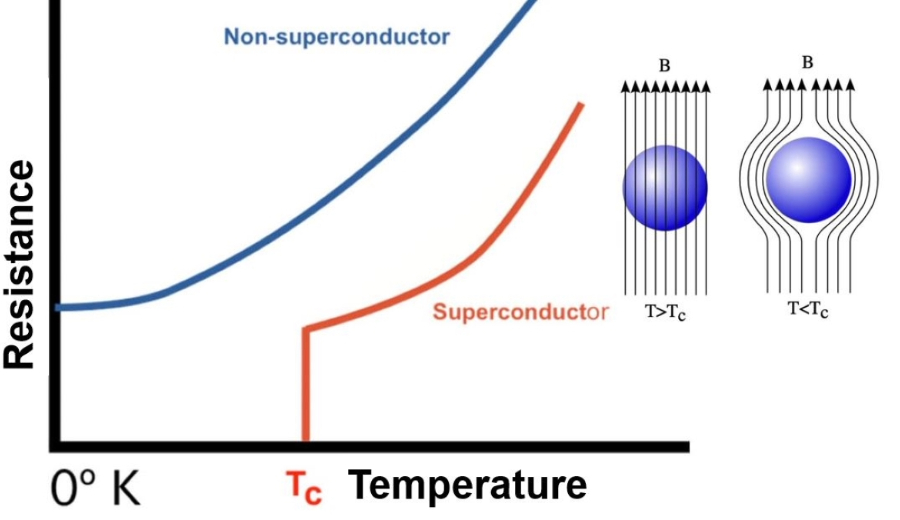
All Electrical Conducting Materials offer their inherent resistance while conducting electricity. Customizing the physical state of materials alters the resistance offered by materials and it was first found by The Dutch physicist Heike Kamerlingh Onnes when he was studying the properties of mercury. On cooling mercury below -268.95 degrees Celsius, its electrical resistance disappeared. The material was able to conduct electricity without heat generation and heat loss. Experimental confirmation by passing an electric current through the cooled sample of mercury indicated that the current continued to flow without any dissipation even after disconnecting the battery. Materials can infinitely conduct a direct current without losing any energy as long as they stay in the same state, such materials are called superconductors.
Superconductivity is basically a low-temperature phenomenon. At low temperatures, electrons either form loosely bound overlapping pairs or glide through the material unhampered to conduct electricity. There is no scientific catch to predict how cold the material should be to conduct electricity with zero resistance. We are able to define entropy, but materials in pure crystalline form do not even behave as superconductors at absolute zero temperatures.
Controversial claims are inexhaustible Hydrides under extreme pressures, metals near absolute zero temperature, and material under the influence of a weak magnetic field are claimed to exhibit superconductivity. Even superconductivity is observed in extra-terrestrial objects like meteorites, which are likely to have been formed under intense conditions.
Recent claims by researchers across the world include yttrium super-hydrides superconducting at temperatures 53 degrees Celsius, and carbon–hydrogen–sulfur compound becomes superconducting at 15 degrees Celsius. Currently, the highest superconducting temperatures at ambient pressure are around –138 degrees Celsius (135 Kelvin), found in “cuprate” superconductors, a family of copper-containing compounds discovered unexpectedly in 1986. Electron pairing in the cuprates appears to involve a different mechanism than interaction with the lattice. However, no theoretical proof is available to explain how material conducts electricity with zero resistance and also limiting temperature to achieve the same.
Exploration for superconducting materials progressed to the extent that we started probing for room-temperature superconductivity. A material that superconducts at everyday temperature and pressure could be used much more widely for two reasons. First, we can’t create extreme pressures or lowest temperatures to convert a material into a superconductor across transmission lines by expensive energy, which effectively means spending energy to save energy. Second, developing a material under extreme conditions to minimize energy losses by using energy is also not a wise option.
Though century-old progress is a piece of evidence that we can achieve superconductivity under ambient conditions in the near future. Once it is achieved, the entropy of the universe may not be increasing at the current rate as low entropy energy generation will cease to exist and energy can be transformed without losses.
Source
- https://news.mit.edu/2023/simple-superconducting-device-could-dramatically-cut-energy-use-computing-other-important-0815#:~:text=That’s%20because%20 superconductors%20transmit%20current,in%20the%20form%20of%20heat.
- energy.gov/science/doe-explainssuperconductivity
- https://www.bitsathy.ac.in/wonders-of-lk-99-superconductors/
- https://energyeducation.ca/encyclopedia/Energy_loss#:~:text=When%20energy% 20is%20transformed%20from,form%20of %20energy%2C%20like%20heat.
- https://www.chemistryworld.com/superconductors/1618.tag
- https://www.thehindubusinessline.com/news/science/is-superconductivity-finally-within-reach/article67161156.ece
- https://www.thehindu.com/sci-tech/science/superconductivity-stay-in-the-flow/article67216178.ece